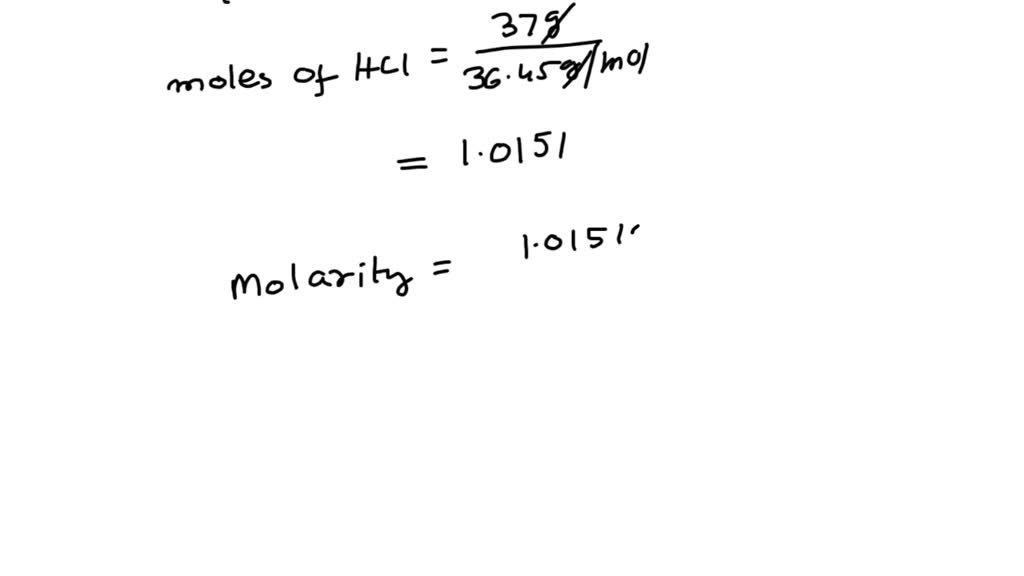A 500 ml 0.2 M HCl solution is mixed with 500 ml of 0.1 M NaOH solution. What is the concentration of [H+] in the final solution? - Quora

The volume of 0.1M HCl required to neutralize completely 1g of an equimolar mixture of {Na}_{2}{CO}_{3} is:318.76mL215mL325mL157mL
31 0.1 ml 0.001 M hcl solution is diluted with water to make 10 litres .calculate PH of the dilute solution.

0 1 M HCl (200ml) and 0 1M NaOH(800ml) was mixed in a container Concentration of OH^- ION IN - Chemistry - Some Basic Concepts of Chemistry - 13700541 | Meritnation.com
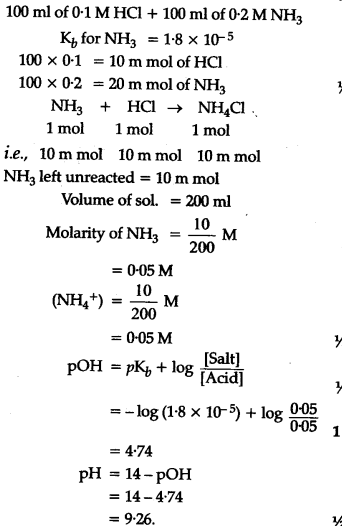
Calculate the pH of a solution obtained by mixing of 100 ml of 0.1 M HCl and 100 ml of 0.2 M - CBSE Class 11 Chemistry - Learn CBSE Forum

Reagents and conditions: (a) 10% NaOH, MeOH, reflux or 0.1 M HCl-EtOH,... | Download Scientific Diagram
final pH of the solution obtained on mixing 100 ml 0.01 M HCl and 100 ml 0.01 M CH3COOH (Ka= 10^ 5) is [log 5=0.7]
To a 50 ml of 0.1 M HCl solution, 10 ml of 0.1 M NaOH is added and the resulting solution is diluted to 100 ml. - Sarthaks eConnect | Largest Online Education Community

L-Lysine Hydrochloride Amino Acid 100mM 0.1 M HCl - Laboratory Research Solution | Procurenet Limited